Risperdal Lawsuit Awards 2.5 Million to Autistic Man
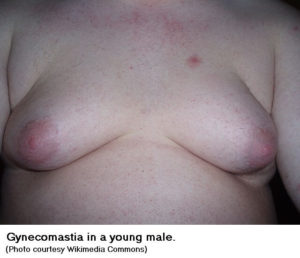 Johnson & Johnson, the maker of the anti-psychotic Risperdal (risperidone) will have to pay 2.5 million to an autistic man in Pennsylvania who grew breasts while taking the drug. The condition, gynecomastia, is a known side effect of the medication, but the company was found negligent in warning both the prescribing doctor and the patient of this potential problem.
Johnson & Johnson, the maker of the anti-psychotic Risperdal (risperidone) will have to pay 2.5 million to an autistic man in Pennsylvania who grew breasts while taking the drug. The condition, gynecomastia, is a known side effect of the medication, but the company was found negligent in warning both the prescribing doctor and the patient of this potential problem.
It’s not the first payout from J&J, nor is it likely to be the last. The now-20-year-old man suffers from autism, and one of the indications for the drug is treating the irritability and “acting out” common with the condition. However, the mechanism of action – altering brain chemistry – also triggers the release of prolactin, a hormone known to be involved in normal growth of the breast, both in adolescent girls and during pregnancy. This hormone is not normal in males, but their bodies respond by growing breast tissue nonetheless, including fat deposition and the glands that allow for lactation.
The victim in this case grew size 46 DD breasts while on the drug.
Drug-induced gynecomastia, in more severe cases, requires surgery to correct. But the emotional scars are perhaps the more serious tragedy for a young man, especially when he also suffers from autism. The difficulties of facing the world with a mental disorder are only magnified by the bizarre appearance, especially during the teen years, when peer approval is so important.
A History of Problems
Last month’s jury award follows a string of settlements involving Risperdal. There were a series of cases against the company for another, sometimes fatal, side effect when the anti-psychotic was used to calm patients with senile dementia. Elderly patients were found to have an increased risk of death from various causes when placed on this class of drugs. The company now warns against using their product for dementia-related psychosis.
In 2012, J&J settled a group of criminal and civil charges alleging the drug maker was marketing Risperdal to physicians for conditions that hadn’t been studied and for which the drug was not approved. Doctors relied on suggestions from the company about using the drug in adolescents and children before the FDA started allowing it in 2006.
The basis for the FDA approval for use in autistic children is also being criticized, since the company only conducted short-term trials in their submission. This is not unusual, since the drug had already been on the market for decades, and was thought to be safe for use in humans. But developing children are more susceptible to certain side effects. The short-term studies performed did not show the problems which can emerge with chronic use.
Should It Be Used at All?
The choice to medicate autistic children isn’t an easy one. It’s become a polarizing topic among parents with affected children, and the arguments are ongoing. Tempers flare on each side of the issue, and caregivers are caught in the middle – wanting to do the best for their child, but with no clear idea what “best” actually may be.
Autistic children who throw temper tantrums, are violent, or who have sudden mood swings are difficult to care for. In some cases, their aggression towards others, or a propensity to self-harm represent real dangers. It isn’t just about drugging a child to make them more manageable – at least it shouldn’t be.
In this mix, it’s a difficult decision, whether to medicate or not. And the choice of drug has to be weighed against the real possibility of side effects. Parents are faced with a tough choice, one they’ll have to discuss with their child’s doctor. However, not having the full information, information available to the company marketing the drug, destroys the ability to choose wisely. That’s the real damage here – when a corporation puts sales over their responsibility to provide the full risk picture.
What’s Next?
In light of this recent award, malpractice attorneys are being contacted by other parents who’ve had problems with side effects from Risperdal. An avalanche of lawsuits will surely follow. Whether Johnson & Johnson will remove autistic disorder symptoms as a use, or even withdraw the drug from the marketplace is unknown. Since they’ve already put out billions ($2.2 billion in 2013) to pay off other legal actions and keep the drug on the market, they might do the same thing again.
At its peak (2007) Risperdal sales topped out at $4.5 billion. Currently, sales are much lower because the drug went off patent and is available as a generic. Sales for the brand product (and the co-marketed brand Invega – a chemically related derivative) are estimated to be about $800 million globally now.
For those with a child who received Risperdal, either for autism-related problems or for other reasons, the best advice is to consult a Louisville medical malpractice attorney. Gynecomastia is only one of a number of possible side-effects from the drug, and patients who suffered unnecessarily while on the medication deserve compensation.
If you believe Risperdal or any other medication has negatively affected your health, or a loved ones, contact the experienced Louisville dangerous drugs lawyers of Meinhart Smith & Manning PLLC to discuss your situation.
Related Articles